Difference between revisions of "Phospholipase A2 (TG2)"
m |
m |
||
| (5 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{| class="toccolours" border="1" style="float: left; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | {| class="toccolours" border="1" style="float: left; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | ||
| − | ! | + | ! {infobox header}| '''{{PAGENAME}}''' |
|- | |- | ||
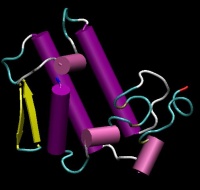
| − | | align="center" colspan="2" bgcolor="#ffffff" | [[Image:PLA2. | + | | align="center" colspan="2" bgcolor="#ffffff" | [[Image:PLA2.JPG|200px|{{PAGENAME}}]] |
|- | |- | ||
| Substrate peptide name | | Substrate peptide name | ||
| Line 24: | Line 24: | ||
|- | |- | ||
| Reactive glutamines | | Reactive glutamines | ||
| − | | [[ | + | | [[TG2 reactive residues (Phospholipase A2)|Q4]] |
|- | |- | ||
| Reactive lysines | | Reactive lysines | ||
| − | |[[ | + | |[[TG2 reactive residues (Phospholipase A2)|K10]] |
|- | |- | ||
| Substrate sequence | | Substrate sequence | ||
| Line 33: | Line 33: | ||
|- | |- | ||
| Structure | | Structure | ||
| − | | [http://www.rcsb.org/pdb/explore/explore.do?structureId= | + | | [http://www.rcsb.org/pdb/explore/explore.do?structureId=1HN4 1HN4] |
|- | |- | ||
| Surface accessibility | | Surface accessibility | ||
| − | | [http://gibk21.bse.kyutech.ac.jp/asaview/all/ | + | | [http://gibk21.bse.kyutech.ac.jp/asaview/all/1hn4A.pdf ASAView] pdf |
| − | [http://gibk21.bse.kyutech.ac.jp/asaview/all/ | + | [http://gibk21.bse.kyutech.ac.jp/asaview/all/1hn4A.txt ASAView] txt |
|- | |- | ||
| Disorder prediction | | Disorder prediction | ||
| Line 46: | Line 46: | ||
|- | |- | ||
| Notes | | Notes | ||
| − | | Transglutaminase probably catalyze the formation of intramolecular isopeptide bonds and this enhances the noncovalent interaction of monomers | + | | Transglutaminase probably catalyze the formation of intramolecular isopeptide bonds |
| + | |||
| + | and this enhances the noncovalent interaction of monomers. | ||
The crosslinking by transglutaminase enhances it's activity. | The crosslinking by transglutaminase enhances it's activity. | ||
|- | |- | ||
| − | | {{infobox header | + | |Video |
| + | |{{#ev:dailymotion|4BZwJSIDhgCWVkY35|300}} | ||
| + | |- | ||
| + | | {infobox header} | | ||
|- | |- | ||
|} | |} | ||
[[Category:Tissue transglutaminase|*]] | [[Category:Tissue transglutaminase|*]] | ||
Latest revision as of 11:51, 10 March 2015
| Phospholipase A2 (TG2) | |
|---|---|
| |
| Substrate peptide name | Phospholipase A2 |
| Synonyms | PLA2 |
| Determination type | In vitro |
| Source | Sus scrofa, Naja naja, Apis sp. |
| Subcellular localization | Cytosol
Venom |
| Swissprot ID | P00592 |
| Reactive glutamines | Q4 |
| Reactive lysines | K10 |
| Substrate sequence | ALWQFRSMIKCAIPG |
| Structure | 1HN4 |
| Surface accessibility | ASAView pdf
ASAView txt |
| Disorder prediction | IUPred |
| Reference | PMID:1976627 |
| Notes | Transglutaminase probably catalyze the formation of intramolecular isopeptide bonds
and this enhances the noncovalent interaction of monomers. The crosslinking by transglutaminase enhances it's activity. |
| Video | 4BZwJSIDhgCWVkY35|300}} |